The production processes of many foods that we buy at the supermarket or eat outside the home can hide a danger to everyone's health. Let's see what it is and how this risk can be avoided or prevented.
What is acrylamide and how is it formed
Acrylamide is a substance with a very simple chemical structure, consisting of 10 atoms including Oxygen (O), Carbon (C), Hydrogen (H) and Nitrogen (N), which are also found in sugars and proteins. It is widely used in various industrial processes to derive thickeners used in the production of paper, permanent press fabrics, dyes, plastics, and in the treatment of white water and waste water (including sewage), in the formulation of pesticides and in tertiary recovery. of oil. Acrylamide derivatives are also found in construction products such as paints and tar, adhesives and packaging.
In the biological field and in scientific research, a water-soluble gel based on polyacrylamide, an acrylamide polymer, is used for the study of proteins. Acrylamide powder is a powerful neurotoxin that can be easily absorbed by the skin. Once gelled in its polymer, it loses its dangerousness as it is no longer absorbable; In any case, researchers must wear protective gloves, goggles and / or suitable masks to avoid inhaling the powder before solubilization.
However, cigarette smoking and some foods are the main sources of acrylamide exposure for the population. Acrylamide is found mostly in the following foods: French fries and packaged fried products (chips, corn tortillas ...), crackers, bread products, rusks and biscuits, cereals, black olives in tin cans, plum juice, coffee and products toasted. The levels of acrylamide in foods are variable and depend on the production method, time and temperature of the cooking processes. Smokers also have 3 to 5 times higher acrylamide levels than non-smokers.
The presence of acrylamide in food was first reported by scientists from the Swedish National Food Authority and the University of Stockholm in April 2002. Researchers initially investigated the exposure of workers in tunnels following the accidental release of agents. for grouting and cementation; however they could not understand why workers not previously exposed to acrylamide (controls) had levels of this compound in their blood. This data prompted scholars to investigate sources of exposure in everyday activities and found that acrylamide formed readily in some carbohydrate-rich foods while its content is reduced in higher protein products such as meat and fish. .
This compound is not found naturally in foods but can form quickly when sugars and amino acids (the "building blocks" of proteins) react with each other in foods rich in carbohydrates and starches during the cooking process, if this occurs at higher temperatures at 120 ° C. Among the amino acids involved, the one that plays a fundamental role is asparagine, present in many starchy vegetables, especially potatoes. The reaction that leads to the formation of acrylamide is known as the “Maillard reaction”, which is also responsible for the crispy crust on grilled meat, bread, pizza, chips, vegetables in batter, etc. The more browned a food appears, the more acrylamide will have formed.
If one of the two components, or sugars or amino acids, is missing, the acrylamide is not formed. High amounts of acrylamide have also been found in potato and beet-based preparations, especially if they are heated: in fact, in these two vegetables, the levels of acrylamide are influenced by the high content of asparagine.
What are the risks to our health?
Exposure to acrylamide depends on the combination of foods consumed in the diet and their method of preparation, smoking habits and passive smoking, professional sources. Skin absorption of acrylamide is low as the skin forms our first barrier against external hazards. Conversely, oral exposure is the main source of acrylamide intake.
In fact, both in humans and in animals, after ingestion, acrylamide is rapidly absorbed in the gastrointestinal tract and, through the body fluids, reaches all organs. The parts of the body where it accumulates the most are the kidney, liver, nervous system, blood and testicles. It can even cross the placenta and is present in trace amounts in breast milk.
One of the main metabolites that are formed in the body is glycidamide, a molecule that is able to bind to DNA or proteins such as hemoglobin in red blood cells. Acrylamide and glycidamide act through the formation of free radicals, responsible for oxidative stress in our cells. Several studies have found that both of these compounds are dangerous toxins for the nervous system. Neurotoxicity in humans is associated primarily with occupational risk rather than dietary and clinically manifested through peripheral neuropathy, numbness, muscle weakness, and difficulty moving.
Exposure of laboratory animals to high doses of acrylamide and glycidamide, administered orally in drinking water, has genotoxic and carcinogenic effects, i.e. it causes DNA damage, causing the onset of tumors in various organs (brain, udder, uterus, testicles, kidneys, skin, stomach, endocrine glands ...); other harmful effects are borne by pre- and post-natal development, as well as negatively affecting the functionality of the male reproductive system.
There are still few human studies to draw definitive conclusions and the results obtained provide only limited evidence regarding the risk of developing cancer following dietary acrylamide intake. Following the discovery of acrylamide in cooked foods, the first food monitoring campaign began in 2007, with the European Commission's Recommendation 2007/331 / EC and the publication of Guidelines to reduce the acrylamide content in processed foods.
Then followed the European Union Recommendations 2010/307 / EU and 2013/647 / EU. In June 2015, EFSA, the European Food Safety Authority, published a report in which it assesses the risks related to the presence of acrylamide and glycidamide in starchy and carbohydrate-rich foods, indicating the need to control their consumption. Experts from EFSA's Scientific Panel have reconfirmed the assessments made in previous years by the World Health Organization (WHO) Expert Committee on Food Additives and the United Nations Food and Agriculture Organization (FAO) according to which the acrylamide taken with the diet increases the risk of genetic mutations and, consequently, of the onset of tumors in all age groups. EFSA also reiterates the need to carry out more studies and research on humans to identify their toxicology, exposure and training.
Finally, in November 2017, the European Commission announced Regulation 2017/2158 / EU establishing mitigation measures and reference levels for the reduction of acrylamide in food, applied starting from 11 April 2018 (Table 1) and mandatory for the food industries. Since acrylamide is a potentially carcinogenic substance, it is not possible to establish its toxic dose as, theoretically, even a single molecule would be enough to trigger a series of events that lead to the development of a tumor in more predisposed individuals. The only toxicological principle currently applicable is based on the reduction of acrylamide to the lowest possible levels.
Low doses can hardly damage health as acrylamide is eliminated through urine and faeces. However, the more risky foods you consume, the more likely it is that acrylamide will become dangerous. For this reason, EFSA's experts have estimated a margin of exposure (MOE), above which acrylamide can cause a slight but measurable incidence of neoplasms and other adverse effects in humans. The MOE then provides an indication of the alarm level. This value is defined as the “lower limit of the confidence interval for the reference dose” or BDML10; for the neoplastic risk, this value must not exceed 0.17mg / kg of body weight per day while, to avoid neurotoxic effects, it must not exceed 0.43mg / kg of body weight per day.
How to avoid or limit the consumption of acrylamide
Since it is present in many foods and is formed at high temperatures, in addition to being used in some industrial processes, it is not possible to completely eliminate acrylamide but some simple measures can be adopted to reduce it to acceptable levels.
- The most important and valid advice for all aspects of our daily life is to maintain a varied and balanced diet, increasing the consumption of those foods that are not at risk, i.e. fruit, vegetables, meat and fish, and instead decreasing the amount of snacks and processed and packaged foods, especially if fried or cooked at high temperatures.
- As for foods with a high carbohydrate and starch content, it is advisable to opt for cooking methods such as steaming, boiling or bain-marie which does not lead to the formation of the brown "crust".
- Acrylamide forms only on the surface of slightly "scorched" foods. When cooking hazardous foods in a pan or oven, just prepare larger pieces to reduce the percentage of acrylamide for the same weight of food eaten. If while eating pizza or grilled foods you notice clearly burnt parts, eliminate them because they are the points of greatest acrylamide concentration.
- Already cooked foods should not be cooked a second time; to heat them opt for the microwave which does not raise the temperatures excessively.
- In the home preparation of baked goods, use as little sugars as possible (sugar, honey and fructose) and prefer natural leavening as the yeast has the ability to lower the pH of the dough, limiting the formation of acrylamide.
- Since potatoes are among the foods richest in sugar and asparagine, it is not recommended to keep them in the fridge as it would favor a greater release of glucose ready to react with the asparagine resulting in the formation of acrylamide during cooking. Potatoes should be stored at room temperature, in the dark, avoiding sprouting them.
- Frying potatoes represents optimal cooking to preserve the Vitamin C, abundant in this vegetable. Just think that 100g of raw potatoes contain about 30mg of Vitamin C which, after frying, remains active up to 80%; a similar quantity can be found in a large fresh apple. However, particular attention should be paid to the frying oil: it should not be reused, especially if some food has remained too immersed in it; furthermore, the smoke point must never be exceeded. For frying it is better to avoid butter or other fats characterized by a low smoke point; the best frying oils are olive, coconut and peanut oil with a very high smoke point.
- Lowering the cooking and frying temperature below 170 ° C, interrupting the process when a slight browning appears instead of a brown color, allows a reduction of acrylamide by almost 90% compared to conventional preparation methods.
- Coffee and its substitutes, due to roasting, contain higher levels of acrylamide than other foods; we must not deprive ourselves of it, it is sufficient to limit its consumption, opting for sweeter blends and alternating the intake of caffeine through for example tea.
Vitamins, antioxidants and natural compounds represent a valid help to reduce the percentage of acrylamide
Free radicals, which are formed following the intake of environmental and food toxins such as acrylamide, are responsible for the oxidative stress which is at the basis of the onset of chronic, metabolic and neoplastic diseases. Antioxidants are substances capable of inhibiting the formation of free radicals in food and contribute to their elimination within our body, detoxifying us.
As already mentioned in point 5, the decrease in pH during the leavening of baked products allows to limit the formation of acrylamide during cooking. To do this, in addition to yeast it is possible to use bicarbonate as a leavening agent; or, the addition of organic acids such as citric acid obtained by squeezing the lemon and the acetic acid contained in the vinegar allow to reduce the levels of acrylamide even in 60%. Obviously, since these compounds have a bitter taste, it is necessary to dose them carefully in order not to compromise the sensory acceptability of the final product.
Scientific studies have also evaluated the effectiveness of adding green tea extracts to the breading of fried products at 175 ° C such as chicken wings and legs, cutlets and chips. Furthermore, green tea extract does not alter the taste of the dish. Interfering with the formation of acrylamide are some flavonoids with antioxidant action contained in tea leaves and in particular epicatechin and epigallocatechin.
In addition to green tea, other aqueous extracts obtained from aromatic plants such as wild oregano, thyme, rosemary or flowering plants such as bougainvillea or spices such as cinnamon help reduce acrylamide levels during cooking up to 62%. The procedure for preparing the aqueous extracts of these plants is simple and there are numerous recipes available on the web; in fact, it is a question of making infusions, as for herbal teas. Once the extract is obtained, in order for the anti-acrylamide effect to take place, it is necessary to immerse the food to be cooked or fried for a few minutes.
Another useful precaution in the preparation of fried foods, especially potato chips and tortillas, consists in the use of oil containing chilli which inhibits the formation of acrylamide up to 77% and at the same time gives a pleasantly spicy taste to our dishes. Vitamins, such as vitamins A, B6, C, D, E, H (or Biotin), and some minerals such as zinc and selenium also reduce the formation of the dangerous compound by 50%. Finally, the turmeric, così come il ginseng, lo zenzero ed il cardamomo possiedono virtù antiossidanti molto potenti in grado di contrastare la formazione di radicali liberi, proteggendo in particolar modo il tratto digerente, più soggetto allo stress ossidativo attraverso l’ingestione di tossine alimentari come appunto l’acrilamide. Tali spezie posseggono un sapore molto gradevole per cui è sufficiente aggiungerle ogni giorno alle pietanze che prepariamo per proteggerci da molti nemici invisibili.
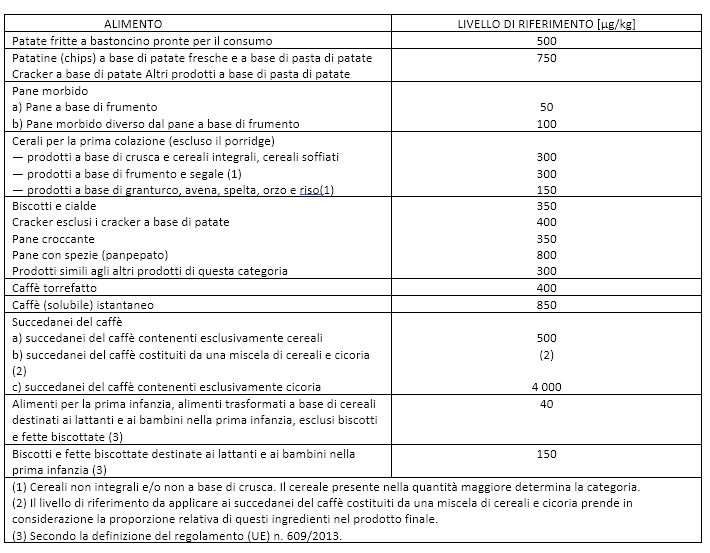
Table 1. Reference Levels for Acrylamide Content in Food 2017/2158.
Bibliography
http://www.salute.gov.it/imgs/C_17_opuscoliPoster_262_allegato.pdf
https://www.fda.gov/Food/FoodborneIllnessContaminants/ChemicalContaminants/ucm053569.htm
https://www.cancer.gov/about-cancer/causes-prevention/risk/diet/acrylamide-fact-sheet
https://www.sciencedirect.com/science/article/pii/S0023643810003798
https://www.sciencedirect.com/science/article/pii/S0278691503003156
https://link.springer.com/article/10.1007/s00003-006-0005-6
https://www.annualreviews.org/doi/abs/10.1146/annurev-food-022811-101114
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4164905/
https://www.cibo360.it/alimentazione/chimica/contaminanti/acrilammide.htm
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4444912/
http://www.raysahelian.com/acrylamide.html
World Health Organization (WHO), 1985. Acrylamide. Environ-mental Health Criteria 49. World Health Organization, Geneva.
Çebi A. Acrylamide Intake, Its Effects on Tissue and Cancer. In: Gökmen V, editor. Acrylamide in Food. Analysis, Content and Potential Health Effects. London: Academic Press, 2016.
Virk-Baker MK, et al. Dietary acrylamide and human cancer: a systematic review of literature. Nutrition and Cancer2014; 66(5):774-790.
Tareke E, et al. Analysis of acrylamide, a carcinogen formed in heated foodstuffs. Journal of Agricultural and Food Chemistry 2002; 50 (17): 4998-5006.
De WT, et al. Influence of fertilization on acrylamide formation during frying of potatoes harvested in 2003. J Agric Food Chem. 2006 Jan 25; 54 (2): 404–408.
Chuda Y, et al. Effects of physiological changes in potato tubers (Solanum tuberosum L.) after low temperature storage on the level of acrylamide formed in potato chips. Biosci Biotechnol Biochem. 2003 May; 67 (5): 1188–1190.
Biedermann-Brem S, et al. How much reducing sugar may potatoes contain to aviod excessive acrylamide formation during roasting and baking? Eur Food Res Technol. 2003 Apr 7; (217): 369–373.
Capuano E and Fogliano V. Acrylamide and 5-hydroxymethylfurfural (HMF): a review on metabolism, toxicity, occurrence in food and mitigation strategies. LWT Food Sci Technol. 2011; 44: 793-810.
Jin C, et al. Relationship between antioxidants and acrylamide formation: a review. Food Res Int. 2013; 51: 611–620.

