Paracetamol (or acetaminophen) is one of the most popular and marketed drugs around the globe.
In Italy it is known under the trade name Tachipirina®, Acetamol® or Sanipirina®, but it is also available as a generic drug or in combination with other active ingredients for use during flu events (stuffy nose, fever and bone pain).
The spread of paracetamol is due to its effectiveness in lowering the body temperature in case of fever and its safety during its use at the doses recommended by doctors, in fact it is administered to millions of people every day, including children and the elderly.
Not everyone knows that in reality paracetamol, administered in high doses, causes a characteristic toxic manifestation affecting the liver and kidneys, which can even be fatal.
During the past 10 years, acetaminophen overdose has been the leading cause of hospitalization for drug-induced liver failure in the US and Great Britain.
About half of the overdoses recorded in the US are caused by suicide attempts, but cases of unintentional poisoning are also on the rise. Prompt intervention and administration of antidotes can save the patient.
Up to 4 grams per day, the dose of paracetamol is considered safe, while for antifebrile and pain relieving therapy, 325-650 mg is given every 4-6 hours. For prolonged therapy, doses higher than 2.6 grams per day are not recommended. The toxicity of paracetamol occurs starting from acute doses of the drug greater than 10 grams. Acute doses greater than 25 grams of the drug can be fatal.
Toxic syndrome occurs approximately 24 hours after ingestion with malaise, headache, anorexia and vomiting.
In case of mild intoxication the symptoms last about 10 days until they disappear.
In case of severe intoxication the symptoms are more severe and a typical paracetamol toxic syndrome is established, whose course can be divided into 4 phases:
- Phase 1: asymptomatic patient or with modest general malaise, oliguria or nausea may appear (0-24 hours after ingestion);
- Phase 2: improvement of general symptoms, mild liver pain.
- Phase 3: it manifests itself after 2-3 days from the administration of the toxic dose with sudden hepatic insufficiency. This is followed by DIC (disseminated intravascular coagulation), following the release of coagulating substances deposited in the liver; hypoglycemia; metabolic acidosis; cardiac arrhythmias and gastric haemorrhages. Renal failure (anuria) also occurs in 10% of intoxicated persons. In the worst case, death occurs after 3-10 days of agony.
- Phase 4: if the patient does not die, hepatic functions slowly recover, thanks to the regenerative capacity of the liver. The damage is visible even after months of intoxication.
Action of paracetamol
Paracetamol is not considered an NSAID (Non-Steroidal Anti-Inflammatory Drug), as it has no anti-inflammatory effect.
Paracetamol works by interfering with pyrogens. Pyrogens are a heterogeneous set of substances that cause a rise in temperature in the body, acting mainly in the body's thermoregulation center, located in the thalamus. For example, inflammatory mediators such as cytokines released by leukocytes in case of inflammation and residues of bacteria killed by our immune system belong to this category of substances.
The mechanism underlying the antipyretic mechanism of paracetamol has not yet been fully understood, it is hypothesized that the drug competes with thalamic receptors sensitive to plasma pyrogen concentration, or by inhibiting the formation of prostaglandins in the central nervous system. The pain-relieving action of paracetamol seems instead to be due to the antagonist effect against bradykinin, a mediator of the pain impulse, but also to the blocking of the enzymatic isoform COX-3 (cyclooxygenase-3), responsible for the production of prostaglandins with effect hyperalgesic; while aspirin, salicylates and all the other NSAIDs selectively block the enzymatic isoforms COX-1 and COX-2, responsible for the production of proinflammatory prostaglandins.
Paracetamol toxicity mechanism
Paracetamol, having a different mechanism of action from NSAIDs, does not cause typical and common side effects on the mucous membrane of the stomach and duodenum such as gastritis or ulcers. These disorders are in fact related to the inhibition of the COX-1 enzyme, a constitutive enzyme, responsible for the production of prostaglandins useful for the protection of the gastric wall. However, an overdose of acetaminophen can be very life-threatening.
The mechanism of toxicity is interesting and involves the metabolism drugs, a physiological process that has the purpose of transforming foreign substances into by-products that are easier to eliminate by the kidney; the metabolic transformation of paracetamol is the cause of toxicity and will be briefly explained.
Paracetamol undergoes rapid first-pass metabolism when taken orally, reaching the liver as early as 2 hours after ingestion. Inside the liver, paracetamol undergoes junction reactions, through which it binds to bulky and very soluble molecules in water, with the aim of facilitating its transport into the blood and expulsion through the kidneys. The two main reactions of conjunctions are:
– sulfation (52% of the amount of drug taken);
– glucuronidation (42% of the amount of drug taken).
Approximately 2% of paracetamol is excreted in the urine unchanged. Finally the remaining 4% comes biotransformed of cytochrome P450 enzymes. Cytochromes are a set of inducible enzymes mainly present in the liver, kidneys and lungs, which inactivate toxic or foreign substances that come into contact with the body through oxidation reactions.
Accidentally, in some cases, the chemical transformations caused by the liver cytochromes activate molecules that were originally harmless to the organism, into toxic substances!
This is precisely the case of paracetamol, in fact in the liver the oxidation of the drug creates an extremely unstable compound: theN-acetyl iminoquinone. Normally this product is detoxified by a further metabolic reaction through conjugation with the glutathione, a scavenger of radical books and other toxic substances. The detoxification processes of drugs create an enormous energy expenditure and the depletion of stocks of antioxidant substances such as glutathione, which in turn must be regenerated through further and complex biochemical reactions; but the toxicity of paracetamol continues because in case of ingestion of large doses of paracetamol, the liver stores of glutathione are depleted before it can be produced again, so the N-acetyl iminoquinone compound will look for other substances to bind to, finding them in proteins present within hepatocytes, the liver cells. The irreversible link between N-acetyl iminoquinone and proteins produces cell death and hepatic necrosis.
Alcoholics and smokers have a more developed cytochrome system than the average, due to the chronic exposure of the liver and the body to foreign substances that must be disposed of daily, such as ethanol, nicotine and tobacco combustion residues. Therefore this path of metabolization of paracetamol is more developed than the first two of conjugation, on the contrary not inducible.
Inducible enzymes are enzymes that are produced in increasing quantities following chronic exposure to toxic substances. Ultimately alcoholics and smokers show symptoms of paracetamol toxicity at lower acute doses than non-smokers and those who use moderate amounts of alcohol.
The antidote for paracetamol toxic syndrome isN-acetyl cysteine: administered intravenously, it provides the toxic by-product N-acetyl iminoquinone with the sulfhydryl groups necessary to restore its chemical stability without affecting the liver proteins when glutathione is depleted.
History of paracetamol
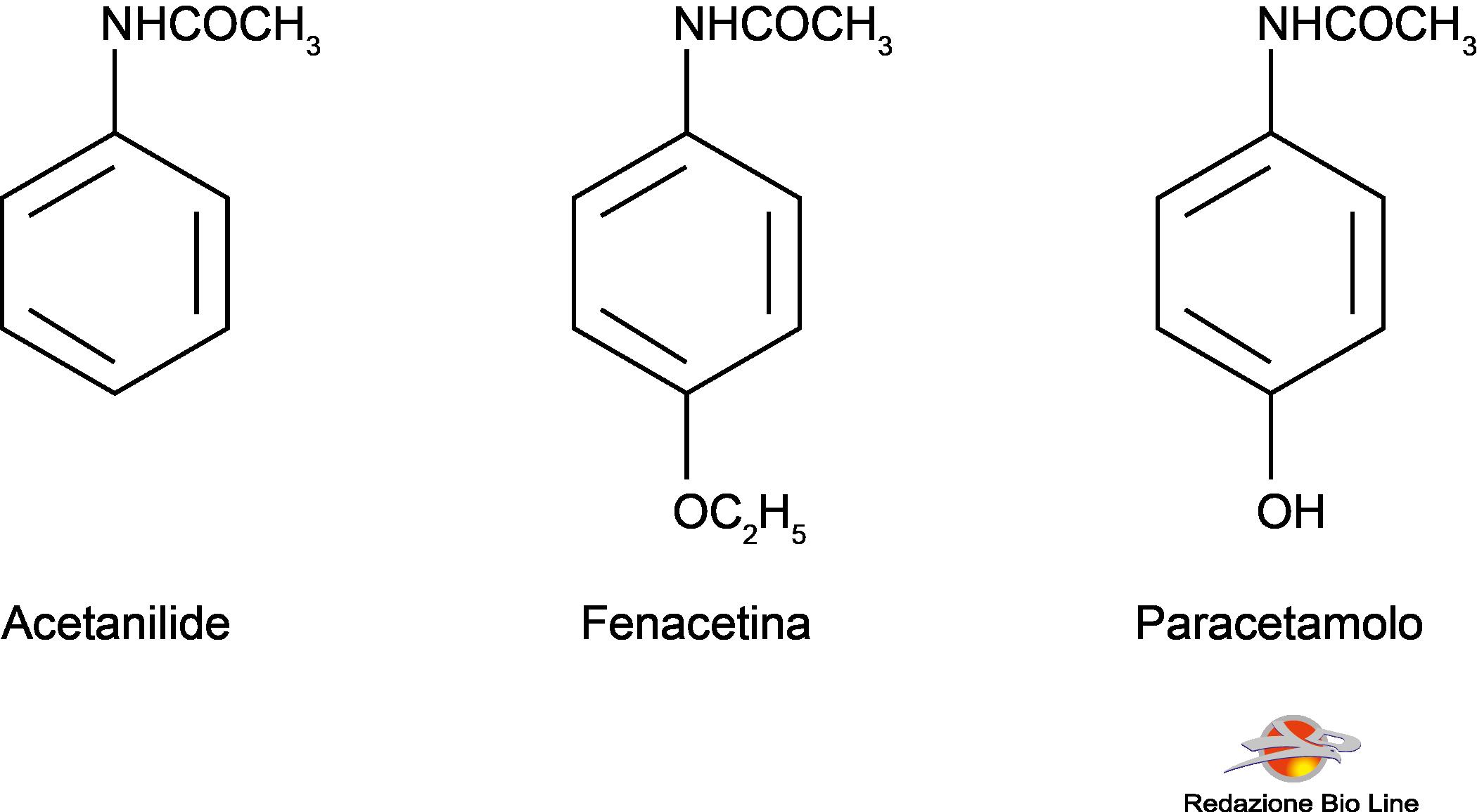
In 1886 it was introduced in therapy as an analgesic-antipyretic theacetanilide, known as antifebbrina; it was later found that it was too toxic and caused methemoglobinemia and jaundice, especially at high doses. The following year the phenacetin, which was used for many years. It too has been withdrawn from the market on suspicion of causing nephrotoxicity. Phenacetin compared to paracetamol has a longer duration of action, as it is metabolized by the body and transformed into paracetamol.
Paracetamol was introduced in 1893, but was neglected for over 50 years, until it was recognized as the metabolite of both acetanilide and phenacetin. He is now the only one left of his group of drugs.
The fever
Fever is an increase in temperature produced by an alteration of the thermoregulatory center located in the brain, in particular in the anterior hypothalamus, due to the presence of chemicals called pyrogens, which can be of different nature:
– exogenous (bacteria, viruses and toxins);
– endogenous (interleukins, interferons and other inflammatory mediators).
Normal body temperature is maintained by the thermoregulatory mechanisms of our body around 36.5 ± 0.7 ° C; a body temperature over 41 ° C (for example in case of heat stroke or malignant hyperthermia), causes denaturation of enzymes, alteration of cell membranes and interruption of mitochondrial functions, the outcome is lethal.
Fever is a defense mechanism activated by our body following infection by harmful microbes; in fact, an increase in temperature creates an unfavorable environment for the replication of bacteria and viruses, which can thus be eliminated more easily by our immune system; moreover, the increase in temperature induces our body to produce interferon and other substances useful for fighting viruses.
However, fever causes a strong sense of malaise and discomfort, headache and nausea, and we are strongly induced to take antipyretic drugs to reduce body temperature, but in doing so we cause a drop in temperature, favoring the replication of the microbes that infest our body; for this reason it is advisable to take antipyretic drugs when the temperature exceeds 38-38.5 ° C.